Given the news (reported by Reuters December 3, 2024) that China is banning exports of gallium, germanium, and antimony to the US, this bonus post is intended to give an overview of antimony and its uses.
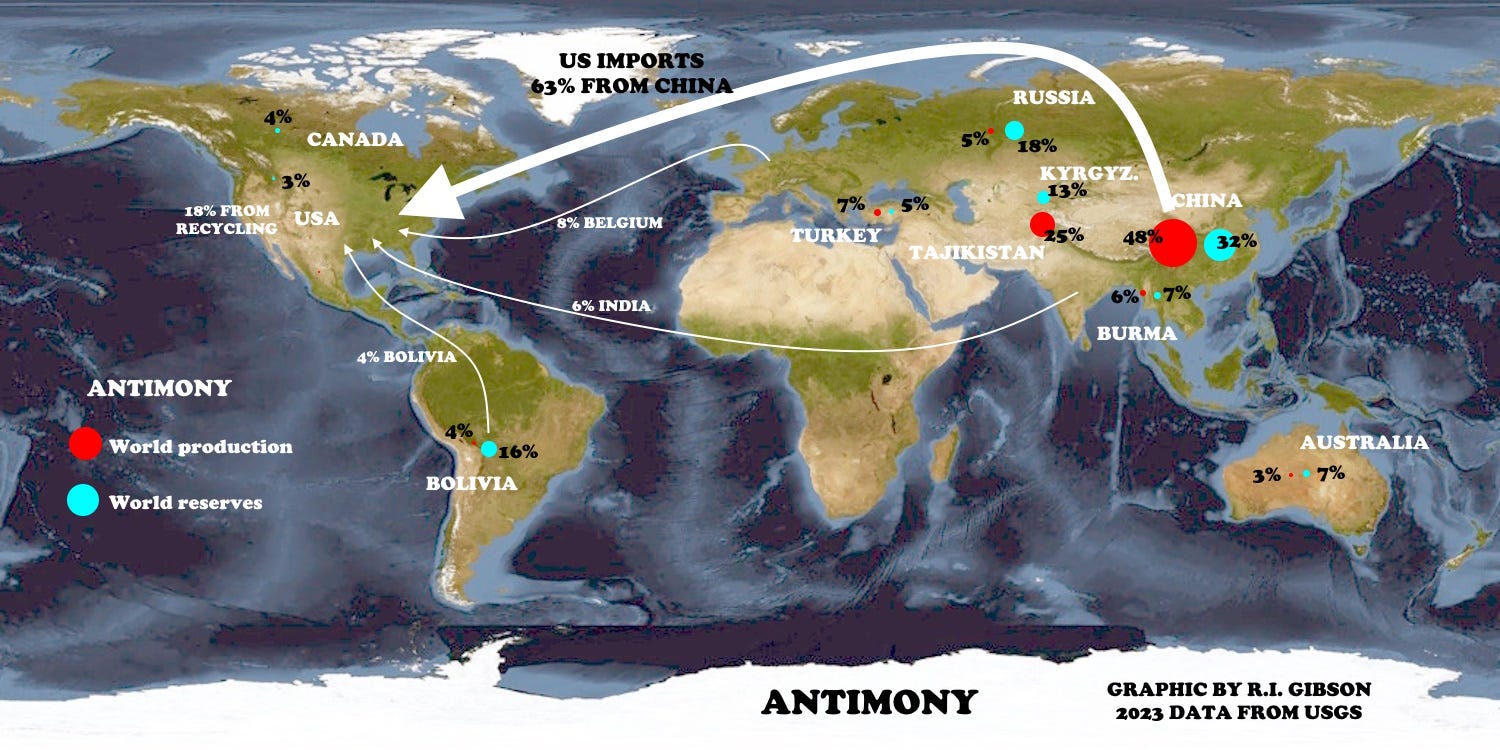
The graphic at the top really says it all: The US is 82% dependent on imports for antimony and has no mine production of it. 63% of US antimony imports come from China, and according to the reports, such imports are to cease immediately.
The price of antimony had already nearly doubled in July-August 2024 because of restrictions on exports by China, from an average of about $5.40 per pound in 2023 and early 2024, to $10 per pound in late August. At the end of November 2024 (last week as I write this) the price was nearly $18 per pound and expected to continue to increase.
So what?
Antimony is vital. The largest deposit in the US, at Stibnite, Idaho, was credited (together with associated tungsten) with saving a million American lives during World War II: "In the opinion of the munitions board, the discovery of that tungsten at Stibnite, Idaho, in 1942 shortened World War II by at least 1 year and saved the lives of a million American soldiers," according to the March 7, 1956 U.S. Senate Congressional Record.
Those lives were saved because of the flame-retarding properties of antimony trioxide combined with chlorine or another halogen. During the war it was applied to tents, canvas jeep and truck tops, and more; today it’s in mattresses, toys, plastics, seat covers in cars, among many other places.
Stibnite, which gave its name to that deposit, is the primary ore of antimony. It’s antimony sulfide, Sb2S3, where the element symbol Sb is from Latin stibium and Greek stibi, their name for the mineral. Despite the fact that only a handful of countries are important antimony producers, stibnite is found as collectible minerals in many localities – at least 50 nations are represented in the stibnite photos on MinDat, but about a sixth of them are from China, like my photo above.
The deposit at Stibnite, Idaho, is reported by the current developer to contain about 67,000 metric tons of antimony, yielding the 3% number for US reserves in the world on the map at top. Perpetua Resources plans to work on remediation of the historical environmental damage in the area as part of their mining operation. The deposit also contains abundant gold. On Sept. 6, 2024, the US Forest Service authorized the mine following an Environmental Impact Statement.
US antimony consumption is about 22,000 to 28,000 metric tons a year, so the mine at Stibnite, with a projected 12-year life, might produce as much as 20% of US demand per year; the US would still be an importer of antimony. Most of the recycled antimony in the US comes from lead-acid batteries, in which antimony helps keep the lead from corrosion and maintains its contacts with battery connectors; that antimony more or less remains within the lead-acid battery industry.
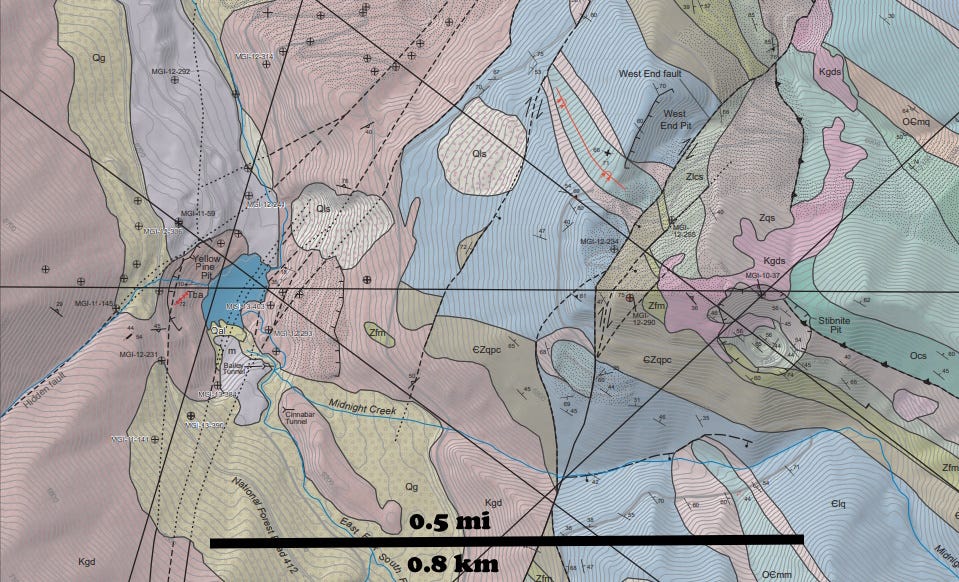
The mineralization at Stibnite, Idaho, is in Neoproterozoic to Early Paleozoic rocks (1,400 to 400 million years old) that sit as roof pendants in Cretaceous (83-94 million years old) Idaho Batholith granitic rocks. The mineralization itself is younger, probably about 60 to 51 million years ago (Gillerman and others, 2019, Geology and Temporal Evolution of Alteration and Au-Sb-W Mineralization, Stibnite Mining District, Idaho: Idaho Geological Survey Bulletin B-31).
Following is an article I wrote about antimony that was originally published in Earth magazine in 1998.
If chemical elements could win Oscars, antimony would get my vote for Best Supporting Actor. Its name comes from the Greek anti and monos, meaning "against being alone." And indeed, both in the ground and in industry, it does its best work in combination with other elements. It's an unsung metal, about as rare as silver, but while it doesn't demand the spotlight, it doesn't shrink from it, either. Actually, it doesn't shrink at all, which is one key to its success.
The main ore of antimony is stibnite, antimony sulfide. The ancient Egyptians and early Hindus ground up stibnite to make a dark eye shadow called kohl. Antimony itself wasn't identified as an element until around 1450, purportedly by an alchemist Benedictine monk, Basil Valentine. Valentine is now usually considered to be a fictional character, invented by a German author, and we're not certain who deserves the credit for isolating elemental antimony, but Nicolas Lemery, a French chemist, wrote the first detailed report on the element in 1707. Early metallurgists learned that, while it wasn't much use on its own, adding it to other metals produced stronger, harder compounds that were more resistant to corrosion. (That's why your car's "lead" battery terminals are actually a lead-antimony alloy.)
Stranger still, they discovered, antimony expands when it solidifies, just as water does when it freezes. Its crystals, like those of ice, contain an unusual amount of empty space - three percent more than do free-swimming molecules in molten antimony. As a result, antimony alloys resist contraction when they harden. This unusual combination of shrink-resistance and toughness means they can be cast with fine edges. An alloy of lead, tin, antimony, and a little copper was the metal of choice for casting movable type for printing from the time of Gutenberg until modern printing techniques superseded "hot metal" a few years ago.
Nowadays much of all the antimony produced is used as a flame retardant in plastics, textiles, rubber, and adhesives. First applied to tents and vehicle coverings during World War II, the fireproofing compound antimony trichloride saved the lives of many American GIs. In a fire, antimony and chlorine recombine to form unstable compounds that suck oxygen out of the air, smothering the flames. Ironically, antimony (without chlorine) also crops up in chemicals used to start fires. Antimony sulfide has weak chemical bonds that cause it to melt and catch fire at relatively low temperatures. For that reason it's a key combustion-supporting ingredient in tracer bullets, smoke screens, "glitter effect" fireworks, and the striking surface of safety matches.
Much of the world's antimony production comes from stibnite mines in Hunan Province of China. Raw stibnite is handsome stuff: bladed clusters of long silvery crystals streaked with long grooves, or striations. The striations look like scratches, but they are actually narrow crystal faces. The finest stibnite crystals come from Shikoku Island, Japan, where magnificent striated blades, some the size of fence posts, crystallized out of hot solutions flowing through cracks in 100-million-year-old rocks. Other fine radiating sprays of crystals are found in China, Romania, and Russia.
In addition to the Stibnite Mining District, a mine in the metal-rich Coeur d'Alene District of northern Idaho historically produced some antimony. There the ore is not stibnite but tetrahedrite, a rare silver-copper-antimony sulfide that yields antimony as a byproduct of refining other, flashier metals. That seems to be antimony's lot in life: always a sidekick, never a star.




This is an excellent report!
Fascinating and all new information for me.
If, God forbid, war were ever to come, how could the U.S function long without antinomy? Is there enough in the Western hemisphere to make up the shortfall?