Here in Butte, America, where I live, last Saturday morning (January 13) the temperature fell to below -40º, a position that’s the same on either the Celsius or Fahrenheit scale. That’s the number at which native mercury takes its place as a legitimate mineral, a solid with trigonal crystallography.
Dihydrogen oxide, another compound that’s technically a mineral only at low temperatures, crystallizes at 40º higher than mercury on the Celsius scale or 72º warmer for us backward Americans using the Fahrenheit scale. But ice is much more common in Butte than native mercury, so here are a few images of its diversity, mostly from within steps of my front door.
The varied crystal forms that ice takes depend on temperature, humidity (water vapor saturation), air pressure, the nature of a substrate, wind conditions, and more. Standard platy hexagonal snowflakes and ice crystals usually form at relatively high temperatures (0º C to -4ºC) and low to moderate water vapor content, and again at lower temperatures (-10 to -20 degrees C).
Columns and needles tend to grow at -4 to -10ºC and colder than -25ºC, but the temperature and geometry of a substrate can exert more control than air conditions. The 5-6 cm rods above are probably thin hexagonal columns that grew on the vertical side window of my car sheltered from the wind, while the sloping windshield in the wind and probably a bit warmer was the site for the feathery ice to grow, although both are present to some extent in both places.
Sastrugi are wind-sculpted ice forms that are largely erosional but can possibly include some recrystallization on the lee side. The word is Russian and was used by both Scott and Shackleton in their Antarctic explorations. These tiny ones (compared to Antarctic and Siberian sastrugi that can be many meters high) are on the street in front of my house.
I gather that solid hexagonal plates like those above can form at almost any temperature, but they usually represent very low water vapor saturations whereas the complex dendritic forms of most snowflakes grow under conditions of higher water saturation (Libbrecht, 2005, The physics of snow crystals: Reports on Progress in Physics 68:4).
I think the 2-cm rosettes above could be considered corn snow, which grows on the ground in partially isolated granular aggregates as a result of multiple freeze-thaw events. I think these in my driveway grew on a relatively warm day (near 0ºC) with warmer breezes traversing an irregular snow surface in the shade.
The regularity and complexity of the negative spaces within this thin sheet of ice is interesting. It’s on the inside of my car windshield.
We get lots of hoar frost in Butte, at least any time from September to May (and the fact that I haven’t seen any in June-July-August certainly does not preclude it forming then; I’ve seen snow every month).
Here there be dragons.
The ice crystals in this halo and sun dogs are too far away to see in detail, but they still have a visual impact.
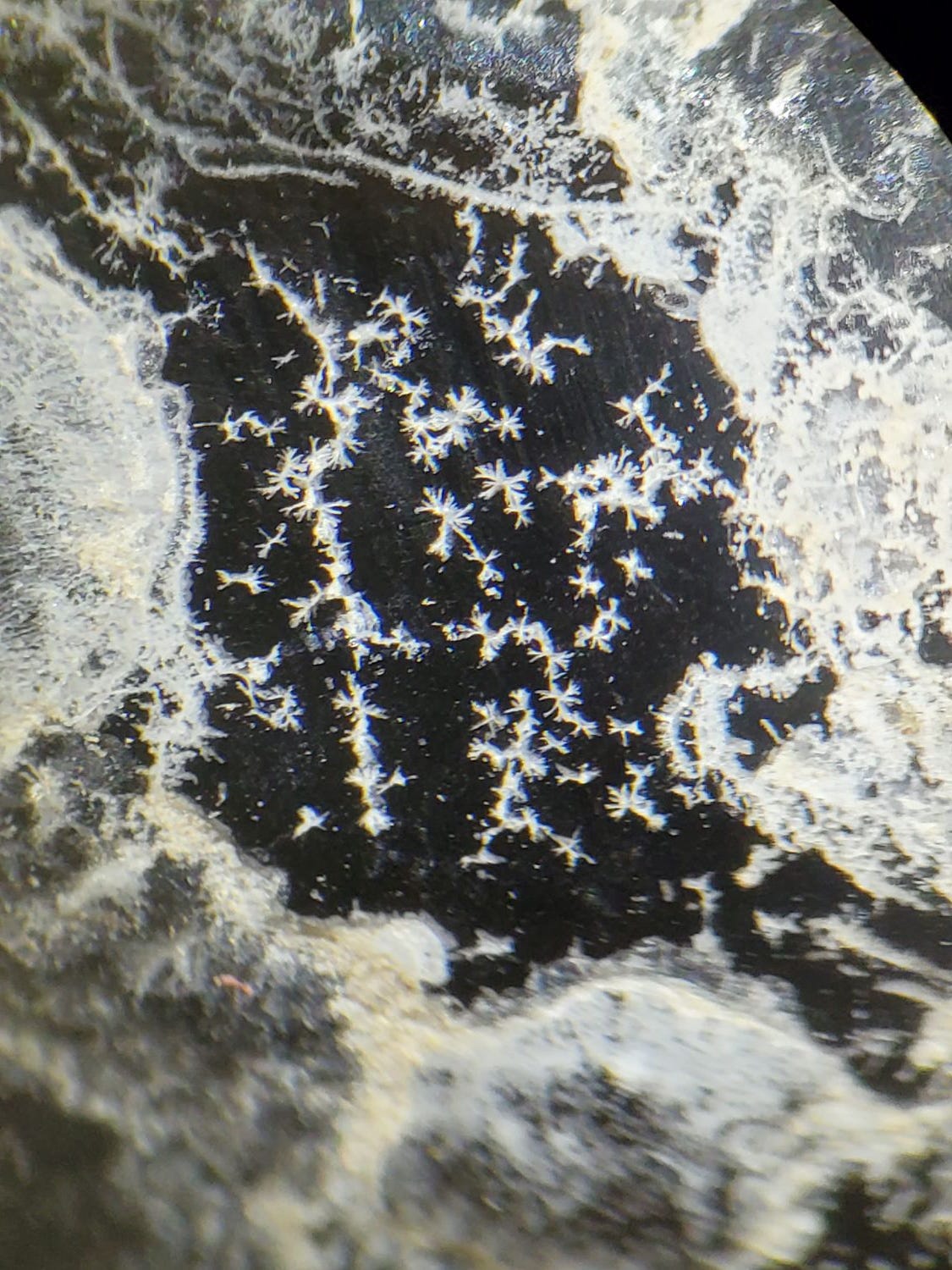
Oops – this one isn’t ice. Those are cristobalite “flowers” growing on obsidian, from Arizona USA.
















Thanks Richard. Setting up some of those shots must have taken some time.
Ice is amazing!
I was once working out on my backyard trampoline one cold winter day; it started to snow perfect flakes, they would land on the black expanse of the trampoline and as I was jumping, it made it look like I was falling/ landing into a starry night sky. I had to laugh out loud it was such a cool effect. When all the right conditions come together...
Outstanding article! As usual.......