
Napoleon’s campaign in Egypt and Syria in 1798-1801 was a military failure, but the expedition brought two things back to Europe of scientific interest: The Rosetta Stone, and geologist Déodat de Dolomieu, for whom the mineral and rock dolomite were named.
The Rosetta Stone is dark, relatively fine-grained granodiorite that was probably quarried south of Aswan, Egypt (Middleton and Klemm, 2003, The geology of the Rosetta stone: The Journal of Egyptian Archaeology, 89:1, 207-216). The granitic rocks around Aswan date to about 622-595 million years (very late Precambrian, Neoproterozoic; Finger and others, 2008, U-Pb zircon ages and geochemical data for the Monumental Granite and other granitoid rocks from Aswan, Egypt: Implications for the geological evolution of the western margin of the Arabian Nubian Shield: Mineralogy and Petrology 93(3):153-183). That’s the time of the Pan-African Orogeny when small cratonic blocks and other materials came together to assemble the supercontinent of Gondwana.
Napoleon’s expedition included an astonishing cadre of 167 scientists, artists, and scholars, ostensibly to promote the principles of the Enlightenment and to do research and survey for a Suez Canal, but some see the scientists as little more than propaganda to obscure Napoleon’s aspirations for world power. In any case, the principal geologist with the expedition was Dieudonné (Déodat) Sylvain Guy Tancrède de Gratet de Dolomieu, one of the world’s most prominent geologists at the time.
Naming the mineral dolomite, magnesium carbonate, was a case of glorifying a prominent scientist who published in noted journals. The mineral was actually first described by Swedish botanist Carl Linnaeus, who in 1768 recognized it as something like calcite but which did not effervesce violently in hydrochloric acid (the standard test for calcite today). He called it marmor tardum, “slow marble,” for the slowness of its reaction with acid.
In 1778, Slovenian polymath Balthazar Hacquet also recognized the difference between calcite and dolomite, which he called lapis suillus, “stinking stone,” possibly for a petroliferous content.
Dolomieu probably knew of both previous studies when he traveled to the Alps in 1790-91 and found extensive outcrops of the material that reacted slowly with acid; he published his observations in the prominent French Journal de Physique. The following year, influential Swiss (technically, Genevan-born, before the confederation of Swiss cantons in 1815) chemist Nicolas-Théodore de Saussure analyzed the material and named it for Dolomieu (Saussure le fils, M de, 1792, Analyse de la dolomite: Journal de Physique, vol. 40, p. 161–173). It is not clear to me whether the Dolomites in the Alps take their name from the common rock that constitutes them, or directly from Dolomieu himself.
Dolomieu (1750-1801) got his name from his birthplace, Dolomieu in Isère, France. I suppose this was to differentiate him, the one from Dolomieu, from all the other people named Déodat (Dieudonné) Guy Silvain Tancrède de Gratet, but I don’t know the origin of the town’s name.

Dolomite, magnesium carbonate, the mineral that geology students learn about in mineralogy labs, is often pink with characteristic saddle-shaped crystal forms. The “saddles” are really just deformed rhombohedrons, the typical crystal form for calcite, calcium carbonate, although sometimes (depending I think on the proportion of magnesium and perhaps iron) dolomite crystals can be as sharp as calcite crystals.
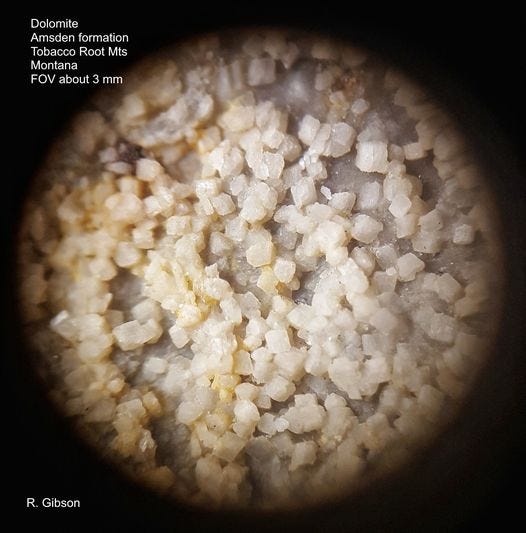
The photo above is dolomite that I collected in 1969 or 1970 above Pole Canyon, near the Carmichael fault zone, in the Tobacco Root Mountains of Montana USA. It’s from the Mississippian-Pennsylvanian Amsden Formation, laid down around 315 million years ago in a variable marine environment that alternated between sandy beaches and carbonate shoals.
The basic rock is a boring chunk of light gray, sandy-textured stuff, so non-descript that I can’t even find it again easily in my house (to be fair, if you haven’t seen my house, there are a couple thousand rocks and minerals out on display, not counting those in boxes, drawers, and the basement). What’s more interesting and informative is this microscopic view that I photographed a few years ago (you may of course perhaps find it equally boring).
Calcite, calcium carbonate, is the main constituent of limestone. It’s very common. Dolomite is almost the same, but there’s some magnesium that substitutes for some of the calcium. You might think, magnesium, wow, rare and cool! But magnesium is really very common too, so dolomite is also common – not as common as calcite-limestone, but still common. Magnesium is the eighth most common element in the earth’s crust and is the third most abundant metal, after aluminum and iron. All the chlorophyll in all the green plants on earth has magnesium in it, where it is vital to photosynthesis (in that, it is somewhat analogous to iron in hemoglobin in blood; hemoglobin and chlorophyll have similar molecular structures).
While we call a rock that’s mostly calcite limestone, we call a rock that’s mostly dolomite dolostone (or often enough, dolomite as a rock name as well as a mineral name. No one said it wouldn’t be confusing and silly.) The magnesium gets into the calcite crystal structure in various situations, some of them pretty subtle. Normal sea water can precipitate dolomite sediment rather than calcite if the conditions of magnesium-to-calcium ratios are right, and/or in certain temperature/pressure conditions, and/or in the presence of catalysts or inhibitors, and other parameters. That happens a lot in sabkhas or salt flats and similar evaporative situations, but that’s by no means the exclusive way dolomite forms. In a solid rock like limestone, if it is permeable, magnesium-bearing solutions can percolate through the rock and react with the calcite to recrystallize as dolomite. Sometimes that happens while the sediment is in the process of lithifying to rock, and sometimes it happens millions of years later.
Dolomitization can be a complicated process, and exactly how it happens has been unclear until recently. This study suggests how primary dolomite can grow through a process of crystal defects and repeated deposition and dissolution.
The key thing that is illustrated in my photomicrograph is that when calcite undergoes the chemical change to dolomite, it also undergoes a crystallographic change, analogous to the development of the saddle shapes mentioned above. When you stick a magnesium ion into the calcite crystal lattice, it warps the shape, so that the resulting crystal is no longer a nice simple rhombohedron but is a bit smaller. Magnesium and calcium ions have atomic sizes that are close – but the difference is enough to make a subtle change in the crystal structure. In a solid rock, that means something has to give: the new dolomite crystals are smaller than the old calcite crystals, on a molecular level, as well as of a slightly different shape. Consequently, in dolomite you end up with extra space between those growing crystals, and you see that in my photo here. The spaces between crystals here have probably been enhanced by natural dissolution, but the porosity is present within the rock, too.
You can also see the crude not-quite-cubic shapes of the dolomite crystals in the photo, and there’s obviously a lot of space between the individual crystals, which are maybe a fifth to a third of a millimeter across. That pore space, the porosity, is probably the primary reason that dolomites and dolomitized limestones are outstanding reservoirs for oil and natural gas. Important fields in the Permian Basin of West Texas, the Williston in North Dakota, and Ghawar Field (the largest in the world) in Saudi Arabia are in dolomite reservoirs. The most prolific onshore oil well in the conterminous United States, in Nevada, produced more than 4,000 barrels a day for 10 or 15 years from a sucrosic (meaning “sugary,” for its fine granular texture) dolomite.
Napoleon’s scientific corps reportedly came under fire at the Battle of Abukir near Alexandria, Egypt, in 1799, but Dolomieu had departed for France four months earlier. His ship had to put into Taranto, Italy, part of the Kingdom of the Two Sicilies, which was at war with France. Dolomieu was imprisoned for the next 21 months but the world scientific community, international politicians including France’s enemies, and the Pope all intervened to have him released. That did not happen until Napoleon conquered southern Italy in 1801 and liberated Dolomieu. But his heath was broken, and he died a few months later at age 51.








A rock composed of mineral dolomite ought to be dolomitite surely?
EXCELLENT -- what a braid of history!