Kidney stones are pathological crystalline build-ups in the human body that are usually minerals identical with those found in nature. A few of the crystalline compounds that may form are organic chemicals, like cystine (an amino acid) and uric acid, and most gall stones are also crystallized organic material, usually cholesterol, but most of the rest are minerals.
When Indiana University’s mineralogist, Carl Beck, died in 1971, his wife asked me (his only grad student at the time) to continue his business analyzing kidney stones. Weird minerals? Sure! Over the next four years, I analyzed more than 20,000 of them.
Beck pioneered the mineralogical analysis of urinary stones using x-ray diffraction versus chemical analysis, which could show for example “calcium phosphate,” but would not show the differences between various calcium phosphate minerals (apatite, brushite, monetite, whitlockite, and others). Different minerals indicate different conditions in the human system and understanding their formation has (sometimes) useful consequences for treatment options. The exact cause of all kidney stones is not well understood, but factors probably include genetics, diet, water quality, and disease.
Disclaimer: I am not a Doctor! Don't use anything here as medical advice. This is intended to provide an introduction to the mineralogy of some pretty obscure minerals, and nothing else!
APATITE is a common mineral in nature. Chemically it is a calcium phosphate with attached molecules of hydroxyl (OH), fluorine (F), and sometimes other elements. Apatite is the fundamental mineral component in bones and teeth, and when apatite has fluorine in its crystal structure, it is stronger. This is why fluorine is added to water and toothpaste. In kidney stones, carbonate (CO3) substitutes for some of the phosphate, making a mineral that is relatively poorly crystallized. Its formula in kidney stones is usually given as Ca5(PO4,CO3)3(F, OH, Cl). Well-crystallized or not, apatite often forms the nucleus upon which other urinary minerals are deposited. It also occurs as a white powdery mineral deposit that can hold other minerals together in a stone.
This oval stone is mostly apatite (plus organic matter tinting it). The thinnest layers I can discern at 40x magnification are less than a twentieth of a millimeter thick. The laminations might represent daily or even more frequent layers of deposition of the mineral apatite in this dog's bladder stone.
WHEWELLITE is a calcium oxalate (CaC2O4.H2O) that is extremely rare in nature. It is known to occur in septarian nodules from marine shale near Havre, Montana, with golden calcite at Custer, South Dakota, and as a fault filling with celestite near Moab, Utah. It is found in hydrothermal veins with calcite and silver in Europe, and it often occurs in association with carbonaceous materials like coal, particularly in Saxony, [former] Czechoslovakia, and Alsace.
But it is one of the most common kidney stone minerals, where it typically occurs as small, smooth, botryoidal to globular yellow-green to brown, radially fibrous crystals. Whewellite stones larger than 10 or 15 mm across are quite unusual, although the larger apatite-whewellite-weddellite bladder stone in the x-ray was close to 3 cm in diameter.
Often whewellite is deposited upon a tiny nucleus of apatite, which may form as build-ups on the tips of tiny papillae in the kidney. Whewellite was named for William Whewell, the British polymath who coined the word “scientist” in 1834 on behalf of Mary Somerville, a diverse Scottish researcher.
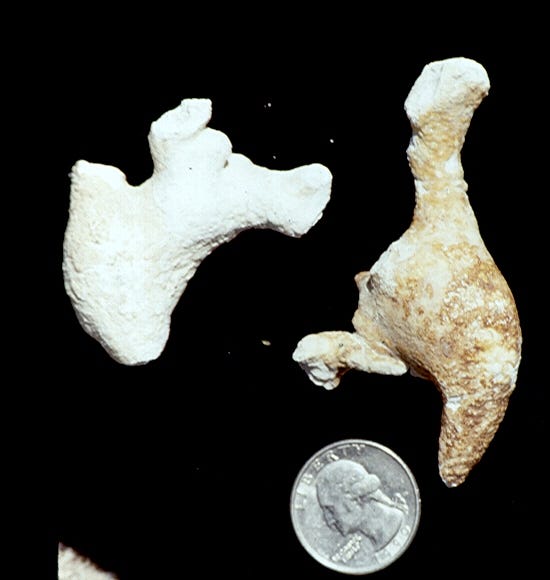
The amazingly huge symmetrical whewellite stone in the top photo was in Dr. Beck’s collection. It’s called a jackstone for its similarity to children’s toy jacks, and while it’s an unusual form, it’s still not that rare for whewellite. The “spokes” of the stone must represent preferential mineral growth on particular, symmetrical crystal positions in the early formed whewellite.
These tiny little beads (that’s a millimeter scale at right) are actually kidney stones. It’s a variety called milk of calcium – the grains are so small that they’re mostly essentially invisible individually without magnification, and just give a “milky” look to the urinary fluid in which they are found. These are probably the common kidney stone mineral, whewellite, calcium oxalate monohydrate.
Why they form disaggregated highly rounded individual grains, rather than the far more common larger layered stones is unknown, at least to me. A reasonable idea is that they nucleated on something, say bacteria, in the urinary system – but while tiny struvite crystals are sometimes associated with Proteus bacterial infections, whewellite is more commonly found in sterile urine. Alternatively, they might have nucleated on powdery bits of apatite, also common in urinary systems.
Milk of calcium seems to be quite unusual (or at least, rarely submitted for analysis) – I only recall about 3 examples out of the 20,000 or so urinary stones I analyzed in 1971-75.
WEDDELLITE, CaC2O4.2H2O, was named for occurrences of millimeter-sized crystals found in bottom sediments of the Weddell Sea, off Antarctica. Unfortunately the sharp yellow crystals that urinary weddellite forms are often much larger than that, and they are frequently the cause of the pain experienced in passing a kidney stone. Rarely, weddellite crystals may occur that are nearly a half inch on an edge, but most are somewhat smaller. The yellow crystals are commonly deposited upon the outer surface of a smooth whewellite stone. Like whewellite, weddellite is a calcium oxalate. They differ in the amount of water that is included in their crystal structures, and this gives them very different crystal habits. Together, apatite, whewellite, and weddellite are probably the most common urinary stones.
Whewellite and weddellite usually crystallize sequentially, with the monohydrate whewellite earlier than the sharp crystals of weddellite. But in this 6-mm example, the short tetragonal weddellite crystals are replaced with grainy, equant monoclinic whewellite crystals, making a pseudomorph (“false form” - the composition of whewellite but the crystal shape of weddellite).
The weddellite crystal highlighted at right is a broken cross-section (the big one at lower right is looking down on the short pyramid faces). There’s one large whewellite crystal highlighted at right, but the entire weddellite crystal there is replaced, so it has dehydrated to whewellite. I can’t say absolutely that this happened in the natural (human) setting versus later (e.g., in the sample container), but weddellite certainly does not routinely dehydrate in dry air – I have some that have been in arid country for decades and are still weddellite.
Magnesium phosphates
STRUVITE is a magnesium ammonium phosphate, Mg(NH4)(PO4).6H2O, that in nature forms distinctive coffin-shaped crystals. Often masses of tiny crystals grow together with powdery apatite to form huge branching stones called "staghorns," which may be several inches long. They may even fill up the entire open area of a kidney. Struvite stones are sometimes associated with bacterial infections of the urinary system. They also require non-acid systems to form, as indicated by the presence of ammonium (a basic, non-acidic compound) in the crystalline structure. The only common occurrence of struvite outside the urinary system is in bat guano. Certain dogs (especially dalmatians) can produce remarkable large, smooth, milky-white tetrahedrons of well-crystallized struvite.
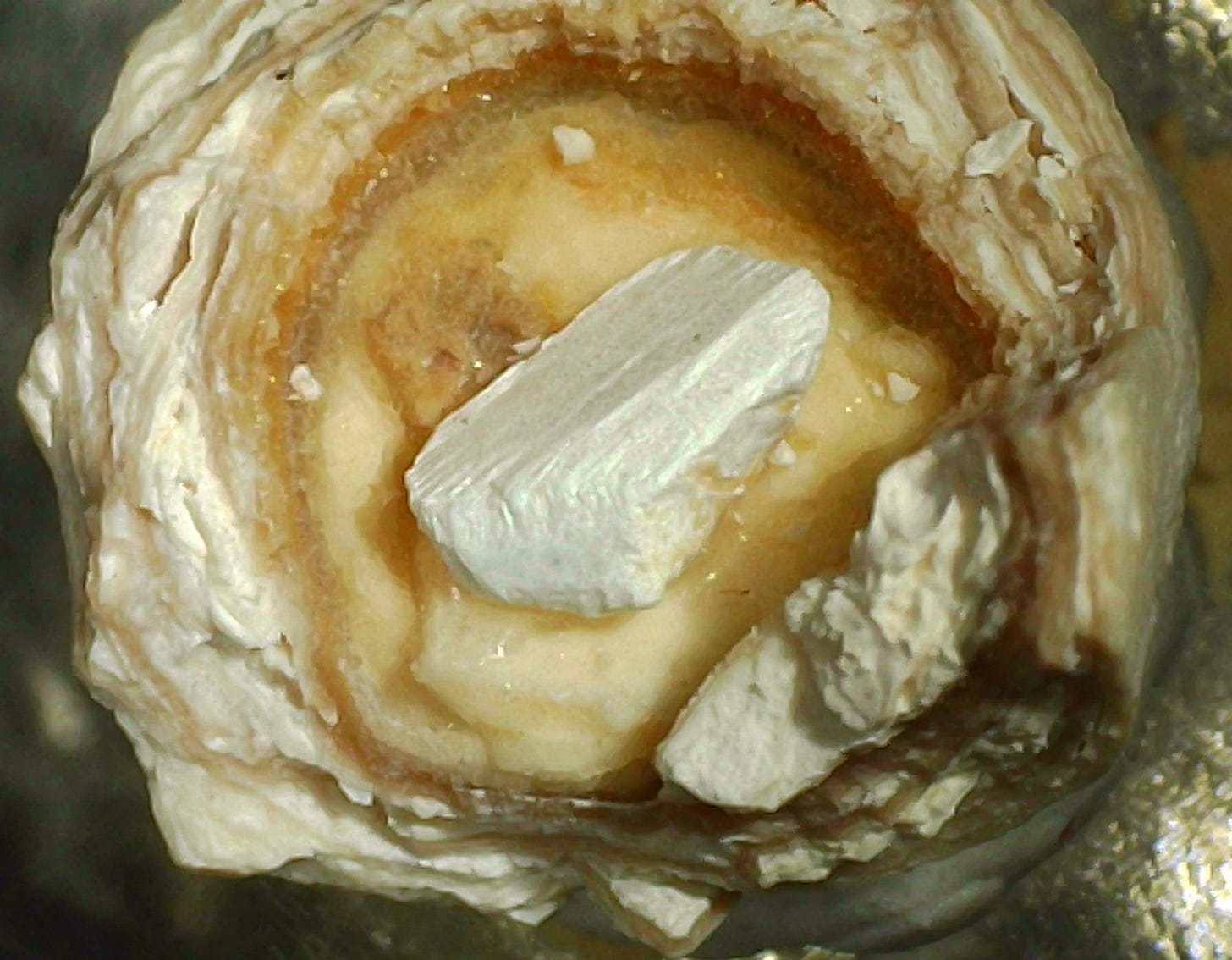
Above: Struvite crystal in a cavity in a banded human kidney stone. The stone is about 6 mm across and is composed of alternating layers of white apatite and light brown whewellite (calcium oxalate).
This kidney stone was originally completely covered by the white struvite crystals, making it look like a grub worm more than 2 cm long. When I went to break it open, my little hammer bounced. The nucleus, a piece of rubber tube, was probably left in during a previous surgery. This was in the days before “malpractice” was a household word.
NEWBERYITE is an acid Magnesium Phosphate, MgHPO4.3H2O (unlike struvite, which contains ammonium) that is rare in kidney stones, probably fewer than 25 out of the 20,000 I saw. When it does occur, it often occurs as tiny isolated globular crystals on the surfaces of apatite-struvite stones. The photo shows light-pinkish purple spherules of newberyite. This probably reflects an alteration of struvite to newberyite, or perhaps a change of conditions to more acidic solutions. Newberyite may be associated with infections of the bacterium Proteus.
The common magnesium phosphate in kidney stones is struvite, Mg NH4 PO4 .6H2O. That NH4 in there, ammonium, indicates that struvite usually crystallizes in basic solutions (as opposed to acidic), and often in the human system that high pH and the ammonium itself result from infections of Proteus bacteria, which liberate ammonia, NH3, which ionizes to the ammonium (NH4+) cation to be incorporated into the struvite crystal structure.
Newberyite’s formula, Mg H PO4 .3H2O, has an “H” (a hydrogen ion) where struvite has ammonia. That tells us it grows in acidic systems, and while slightly acidic urine is normal, it’s unusual for magnesium phosphate to grow in such conditions. This might be because the Proteus bacteria can’t survive, or if they do, the ammonia they create raises the pH so newberyite is no longer stable.
Newberyite was named in 1879 for Australian chemist James C. Newbery (1843-1895) who discovered the mineral and was instrumental in establishing Australian food safety laws. The type locality is Skipton Caves in Victoria, Australia, where it crystallized from bat guano.
HANNAYITE. There’s another magnesium phosphate mineral, also with Skipton Caves as its type locality, hannayite, Mg3 (NH4)2 H4 (PO4)4 .8H2O. In my work on kidney stones, it was identified only 5 times out of 20,000, so it’s really uncommon. Its formula, with both basic (ammonium, NH4) and acidic (H) components, would suggest that hannayite crystallizes very near neutrality, a pH of 7; its rarity may be a result of the difficulty of maintaining such a specific pH in the complex human system. The specimen in the photo is nucleated on a piece of suture. The hannayite is tiny feathery crystals, almost invisible, in powdery white apatite. It’s entirely possible that in the 50 years this has been sitting in my collection it may have dehydrated to some other phase – but it was hannayite when it was analyzed!
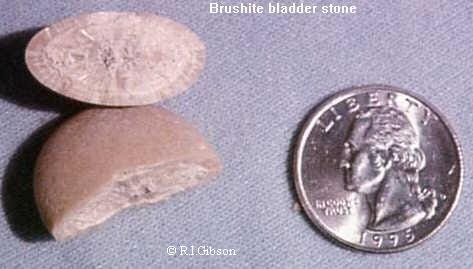
BRUSHITE is a calcium phosphate compound, CaHPO4.2H2O that is very similar to the common mineral gypsum (Calcium Sulfate). Gypsum finds its greatest use in sheetrock and other wallboards used in home construction. Brushite is a rare mineral outside the urinary tract, and even there it probably occurs in fewer than 10% of all stones. It is a soft, silky mineral, usually honey-brown and showing a fine radial fibrous structure. It can only crystallize in a limited range of pH (acidity), so treatment may include changing the acid-base balance of people who make brushite kidney stones.
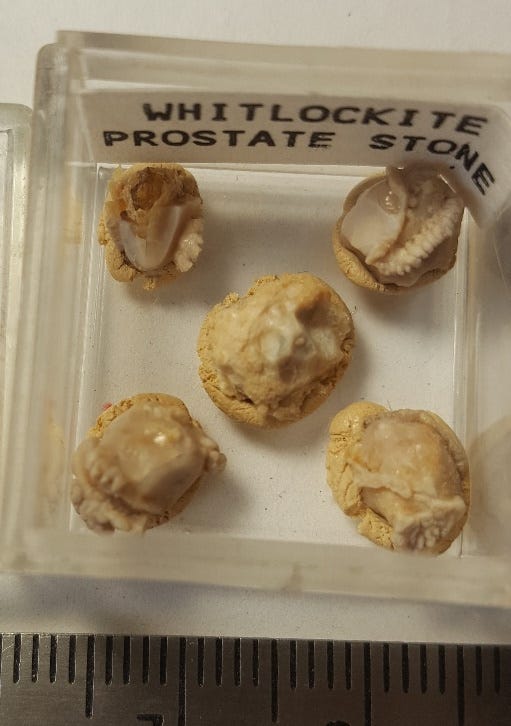
WHITLOCKITE is very rarely found in the urinary system, but it is the most common mineral found in prostate stones. It is a calcium phosphate with magnesium, Ca9Mg(PO4)6(PO3OH), and its occurrence may be stabilized by trace amounts of zinc, and prostate fluid has a very high zinc content. The mineral is a resinous, brown, hackly-fracturing material, and it commonly forms multiple small stones in the prostate.
Other Minerals. Some of the other minerals that occur extremely rarely in kidney stones include monetite (calcium phosphate), calcite (calcium carbonate), and aragonite (calcium carbonate). Chemical analysis routinely reported calcite, but in reality, most of the calcium and carbonate in urinary stones comes from the common carbonate-apatite, calcium phosphate with carbonate substituting for as much as 5% of the phosphate in the crystal structure.
Reference: Gibson, R.I., 1974, Descriptive Human Pathological Mineralogy, American Mineralogist, 59:1177-1182.














I am gobsmacked!!:-)) Just read Steve's post and landed here.. who would have thought all that mineral production goes on in the human body but of course it does! Incredibly fascinating.. thank you for the article. I'm curious, you said "continue his business of analyzing kidney stones.." Who were the customers? Doctors or curious patients?
Excellent article Richard! The Skipton Caves are not far from where I live. Amazing how many minerals occur there that can also occur in the human body.