Life in the USA is not normal. It feels pointless and trivial to be talking about small looks at the fascinating natural world when the country is being dismantled. But these posts will continue, as a statement of resistance. I hope you continue to enjoy and learn from them. Stand Up For Science!
Your science word of the day is thiophilic, from Greek θεῖον, theîon, “sulfur,” plus φίλος, phílos, “love.” Copper is one of the thiophilic, sulfur-loving, elements. It is also called a chalcophile, for its affinity for the chalcogen (ore-generating) group elements, oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). Of those, oxygen and sulfur are the only really important ones (I don’t think there are any polonium minerals, although polonium occurs very rarely in uranium ores). Although “chalcogen” is taken to mean “ore forming,” the “chalco-” part derives from the Greek word for copper, so etymologically, saying copper is a chalcophile is saying copper loves copper.
Copper is pretty common in the earth’s crust, and it makes exploitable deposits because it combines readily with sulfur, also widespread in the crust. At Butte, Montana USA, diverse copper sulfides (chalcocite, covellite, digenite, djurleite), copper-iron sulfides (bornite, chalcopyrite), and copper-arsenic sulfides (enargite, colusite, luzonite, tennantite) dominate the suite of copper minerals.
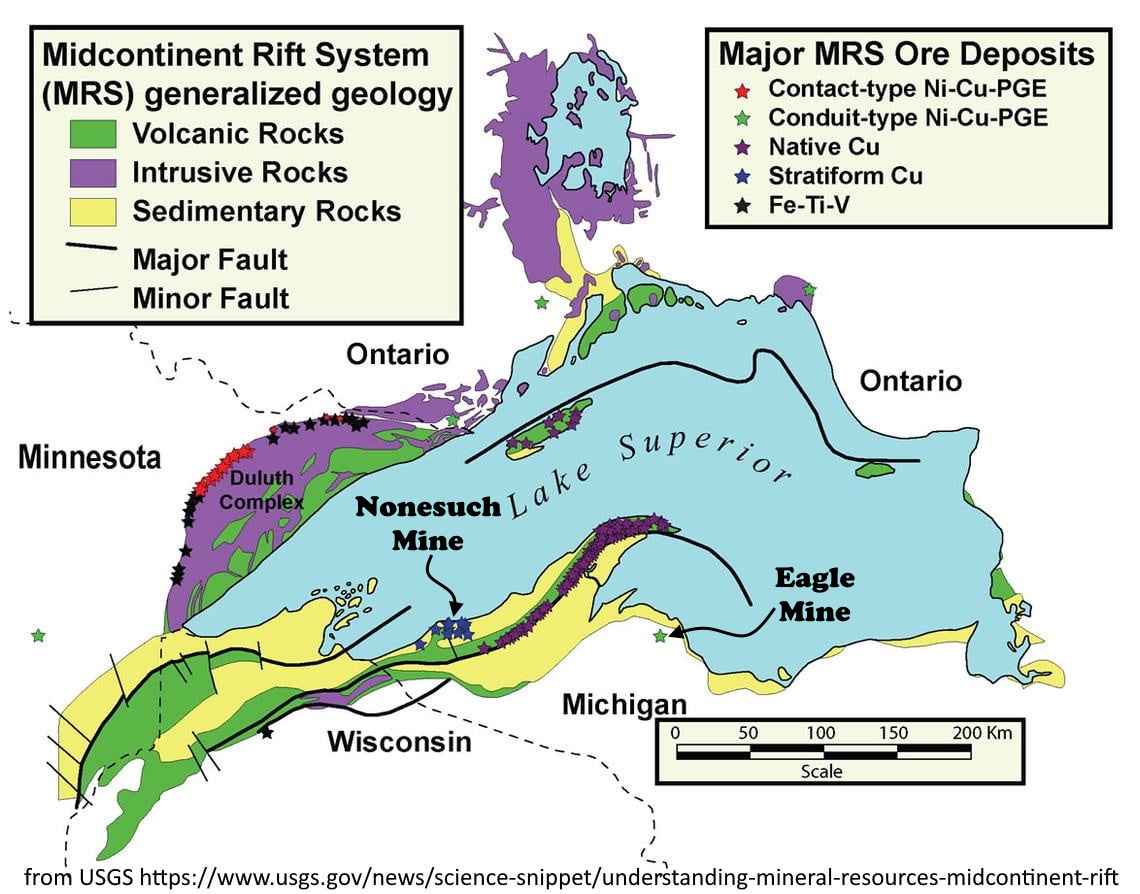
Copper in its pure elemental form, native copper, occurs widely around the world (including at Butte), but not usually in great abundance. Copper WANTS to combine with sulfur. But there is one place, and only one place in the world, where native copper is by far the most common form of copper: the Lake Superior Region, specifically the Keweenaw Peninsula of Upper Michigan and Isle Royale. Why is this so?
The cop-out answer is easy: there wasn’t much sulfur there to combine with the copper, begging the question of why wasn’t there much sulfur present?
The copper in the Keweenaw Peninsula is associated with extensive basalt flows that originated in the Mid-Continent Rift, a 1.1- to 1.06-billion-year-old break in the continental crust of North America that extends in the subsurface from Lake Superior southwest to Oklahoma and southeast beneath lower Michigan. Basalt flows in general have plenty of sulfur associated with them, but elsewhere along the Mid-Continent Rift System basalts are not known to be associated with vast copper deposits, neither native copper nor copper sulfides.
Sulfur might have been removed from the original magma sources. Ding and others (2011, PGE geochemistry of the Eagle Ni–Cu–(PGE) deposit, Upper Michigan: constraints on ore genesis in a dynamic magma conduit: Miner Deposita DOI 10.1007/s00126-011-0350-y) suggest that sulfur might have been depleted in part by early-formed sulfides such as those at the Eagle Mine (see map below), so native copper was favored in later mineralization.
Another possibility is that the magma, even at the time of copper deposition, could have lost its sulfur by degassing just as modern volcanic eruptions put large volumes of sulfur compounds into the atmosphere (Bornhorst, 1997, Tectonic context of native copper deposits of the North American Midcontinent Rift System: Geological Society of America, Special Paper 312, 127-136).
To me, definitely not an expert geochemist, while both ideas have merit, it just seems odd that only once in the history of the earth would such special circumstances eliminate so much sulfur from the system. In particular, degassing would have had to be highly efficient to remove enough sulfur.
I think the current thinking favors the idea that sulfur was already gone from the magma as well as the rocks through which it passed so that native copper was deposited (Bornhorst and Mathur, 2017, Copper Isotope Constraints on the Genesis of the Keweenaw Peninsula Native Copper District, Michigan, USA: Minerals, 7, 185; doi:10.3390/min7100185). Another possibility is that sulfur was progressively removed from the hydrothermal waters that deposited the copper, so that we end up with native copper in one zone and sulfides in another, depending on the oxidation-reduction conditions at various points. But for me, how that sulfur was eliminated is still unclear.
With all that said, the rock I’ve chosen to illustrate native copper from the Keweenaw area is this piece of Nonesuch Shale, one of several units that host the copper.
The Nonesuch formation was probably deposited in a lake that formed within the developing Mid-Continent Rift between 1.083 and 1.070 billion years ago (Strother and Wellman, 2020, The Nonesuch Formation Lagerstätte: a rare window into freshwater life one billion years ago: Journal of the Geological Society, 178:2). In addition to the Nonesuch Shale, the sedimentary sequence deposited in this lake included the Copper Harbor Conglomerate and the Freda Sandstone. It’s fair to think of that lake as analogous to the modern great rift lakes of East Africa – Tanganyika, Malawi, Turkana, Albert, and others – which occupy a volcano-rich rift system that must be similar to the Mid-Continent Rift of 1.1 billion years ago.
Hydrothermal fluids carrying copper from the basalts described above probably entered the Nonesuch Shale and Copper Harbor Conglomerate, where native copper filled the pore spaces – coarse in the conglomerate and finer in the shale, where the copper fills veinlets such as those you see in my specimen. And despite all those words about sulfur above, sulfides, chalcocite and bornite, are also present in the White Pine Mine which is partly in the Nonesuch Shale.
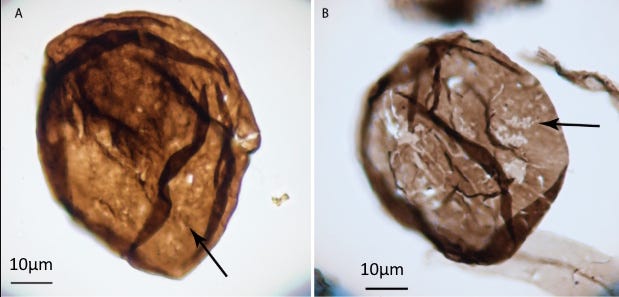
Apart from the copper, the Nonesuch in my specimen is black, because it is organic-rich. The life recorded by this organic material was largely acritarchs, a catch-all name for microfossils of uncertain affinity (the word comes from Greek for confused or undistinguished origin). Most of the examples from the Nonesuch are single cells, quite remarkably well preserved, that may be algae, spores, or egg cases or parts of small multi-celled life. The largest of them are about 70 microns (7 one-hundredths of a millimeter) across, so if there are any in my specimen it would take a microscopic thin section to see them. Despite their tiny size, these acritarchs make the Nonesuch a lagerstätte, a rock of extraordinary fossil preservation (from German for storage place). Strother and Wellman, cited above, described dozens of diverse forms; their entire paper is available online.
The total organic carbon content of the Nonesuch ranges from 0.5% to 8%, averaging around 1%-3% (Fourgani, 2012, Organic geochemistry of Mesoproterozoic Nonesuch Formation at White Pine, Michigan, USA: Master’s Thesis, Colorado State University). This is a phenomenal organic content for a Precambrian rock; for comparison, good Saudi Arabian oil source rocks are around 4% total organic carbon. The lake in which the Nonesuch shale was deposited must have been teeming with microbial life, and it was long but narrow (like the East African lakes today), probably extending from what is now Michigan’s Keweenaw Peninsula southwest into northern Iowa. Live oil with a Precambrian source occurs in the White Pine Mine, but no commercial oil deposits have been found (Seglund, 1989, Midcontinent Rift continues to show promise as petroleum prospect: Oil and Gas Journal, 15 May 1989, p.55-58).
The specimen in the top photo (Cat. No. 308), about 8 cm across, is from the White Pine Mine, Ontonagon Co., Michigan, and it has been cut and coated with urethane to enhance the copper. The lower of the two images is enhanced photographically to show the subtle bedding in the shale, to which the copper veins are subparallel. This was a gift from my parents.
The Nonesuch Formation, a town, and a waterfall were named for the Nonesuch Mine, so called because when the mine began in 1867 there was none such as it in terms of its rich copper deposits hosted in shale and sandstone (or at least the mine operators wanted people to think so). In the long run, that fine-grained copper was difficult to mill and separate from the rock, and the Nonesuch Mine only produced about 200 tons of copper, trivial compared to the more than two million tons of copper the nearby White Pine Mine has produced over time, between a quarter and a third of Michigan’s total copper output. For comparison, Butte’s cumulative copper production is close to 12 million tons.





Fascinating. I never knew about this midcontinent rift. Yet another reason to make my way again to this region!
Visited Upper Michigan when our brother was teaching at Northern Michigan in 1981. Overnight trip to the Keweenaw and Copper Harbor. We walked the beach enjoying pebbles with sparkles of copper in them, but when trying to find some of the old mines back in the woods, clouds of gnats in our eyes and nose demolished our intrepid spirits. Butte will always be "the richest hill", but mineralized UP is fascinating and beautiful. Thanks for this post, interesting that there are organic remains.