Life in the USA is not normal. It feels pointless and trivial to be talking about small looks at the fascinating natural world when the country is being dismantled. But these posts have been scheduled for over a month, and they will continue, as a statement of resistance. I hope you continue to enjoy and learn from them.
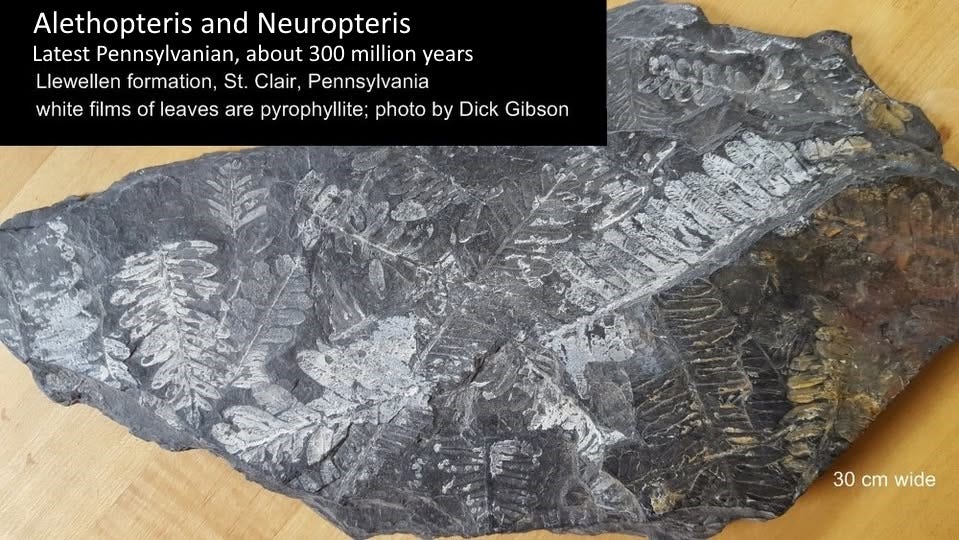
These ferns of Pennsylvanian age from the Llewellyn Formation of eastern Pennsylvania (near St. Clair) include Alethopteris and Neuropteris. The contrasting white of the ferns and the black shaly matrix make for nice fossils. They date to the Virgilian Stage of the Pennsylvanian (corresponding to the Gzhelian of the Carboniferous in European usage), the youngest part of that geologic period, about 304 to 299 million years ago.
But the cool thing about them is their mineralogy and their preservation history. Peterson and others (2011, A multistage model of preservation in fossil plants from the Llewellyn Formation (Pennsylvanian), St. Clair, Pennsylvania USA: GSA Annual Meeting abstracts, Minneapolis, MN) infer a rather complex burial and replacement history for the plants. The leaves were deposited in a clay layer within a package of sediments dominated by coal. Iron sulfide, typically pyrite, is common in anoxic environments like coal swamps, and pyrite may have been the first mineral to replace these leaves. That’s unusual, but not unheard of.
As the sediment pile grew thicker and thicker, temperatures and pressures also increased, and the pyrite was replaced by kaolinite, a soft, hydrous aluminum silicate related to micas. This is the step in Peterson and others’ process I don’t follow: how do you get rid of all that iron sulfide and why would it be selectively replaced by aluminum silicate? All the chemicals are easy to find or imagine in the rock – the aluminum and silica would come from the clays in the shale that encases the leaves. It still seems like an unexpected process to me, but then I’m not that much of a geochemist.
The next step, even higher temperatures and pressures, brought the rocks to a level we’d call early metamorphism, temperatures (and anhydrous conditions) around 300° C (greenschist facies). That changed the kaolinite to pyrophyllite, another hydrous aluminum silicate typical of low-grade metamorphic rocks. The white material in the leaves in this specimen is pyrophyllite with perhaps some residual kaolinite. Apparently microscopic pyrophyllite crystals delicately conform to the geometries of the leaves, resulting in outstanding preservation.
If Peterson et al. (2011) are correct, this outstanding preservation is all the more amazing since it persists despite three different mineralogical replacement phases: pyrite to kaolinite to pyrophyllite.
I believe these pyrophyllite leaf fossils from the Llewellyn Formation are unique in the world, but I’d be happy if some of my paleontologist friends can point out other such examples.
Pyrophyllite is a hydrous aluminum silicate, Al2Si4O10(OH)2. It’s a phyllosilicate, a group that includes micas, clays, and minerals like talc and vermiculite. Phyllo means leaf, a reflection of the sheet-like structures these minerals all have. Pyrophyllite is from Greek words for fire and leaf, an allusion to the fact that it swells and exfoliates (peels) when it is heated, producing masses like sheaves of leaves.
Pyrophyllite typically grows in radiating sprays like the specimen above, so typically that it is pretty much impossible to tell where a piece is from if you don’t know – as I do not know in this case. The most common sources for pyrophyllite specimens in the U.S. are Graves Mountain, Georgia, and a handful of locations in North Carolina and California. All have examples essentially identical to mine, and all are in metamorphic rocks, the most typical setting for pyrophyllite. This one came to me after my Indiana University mineralogy professor, Carl Beck, died in 1971, so it is perhaps likely to be from one of the eastern locations (but he worked in New Mexico and Nevada as well).
Pyrophyllite often forms by low-temperature retrograde metamorphism of high-temperature aluminosilicates like kyanite, but it can also form by prograde metamorphism of clays, as in the case of the fern fossils from Pennsylvania, and by hydrothermal alteration of aluminous rocks including volcanics and sedimentary rocks. It is soft, almost as soft as talc, and has a greasy feel. Pyrophyllite is mined commercially for uses similar to those of talc – refractories in the steel industry and in chimney flues, in paints, and in ceramics including tiles and things like sinks and toilets. It is used as a carrier in some insecticides and in gaskets for some high-pressure applications and sometimes replaces talc as a filler in plastics, rubber, and bricks.
In the United States, the only commercial mining explicitly for pyrophyllite is in North Carolina. Pyrophyllite and talc are seldom reported separately, but for nations that do so, South Korea and Japan are the leading world producers of pyrophyllite, but India produces about a quarter of all the talc and related materials (including pyrophyllite) in the world. China is in second place with about 16% of world talc and pyrophyllite. The US is minimally dependent on imports for talc and pyrophyllite, ranging from 1% to 21% over the time from 2019 to 2023. Most imports came from Pakistan, Canada, and China.
Other posts on “mineralogy meeting paleontology”:






I went on a field trip to the Sandersville kaoline district quite a few years. A point of discussion was why the kaoline was so white. There were people there who were doing research on the role of bacteria in producing such white material. The bacteria would be responsible for somehow carrying off the iron and sulfur that would have been responsible for darker coloring.
Richard, I really appreciate your posts. I am not a geologist but I have a deep interest in geology, minerals and the natural world in general. You are a talented writer with the ability to present 'the big picture' in terms Ms Jo Public can understand. I always look forward to your posts and I always learn something new. Thank you!