Perovskite is a mineral, calcium titanate, CaTiO3. Unfortunately (to my mind) the name “perovskite” has come to have an almost generic use for other compounds with perovskite structure, materials with the general formula ABX3, where A is a metal that forms large cations, typically magnesium, ferrous iron, or calcium; B is another metal that forms smaller cations, typically silicon, although minor amounts of ferric iron, titanium, and aluminum can occur; and X is typically oxygen.
“Silicate perovskite,” as the mineral bridgemanite, (Mg,Fe)SiO3, is probably the most abundant mineral in the earth’s mantle (identified there by changes in seismic properties, and found as inclusions in diamonds derived from the mantle), and consequently, the most abundant mineral in the entire earth.
There are many synthetic perovskites, such as methylammonium lead halide perovskite, which has a high energy conversion efficiency and may see increased future use in solar cells and other applications.
But let’s go back to the first mineral identified to have perovskite structure, calcium titanate, the “real” mineral perovskite. In common igneous rocks, calcium and titanium usually go with silicon to form titanite (sphene), CaTiSiO5. In low-silica melts, perovskite forms, so it is often found in things like carbonatites and low-silica skarns (contact metamorphic rocks), and ultramafic rocks. My examples here are from carbonate skarns and the associated carbonatite intrusion at Magnet Cove, Arkansas, USA.
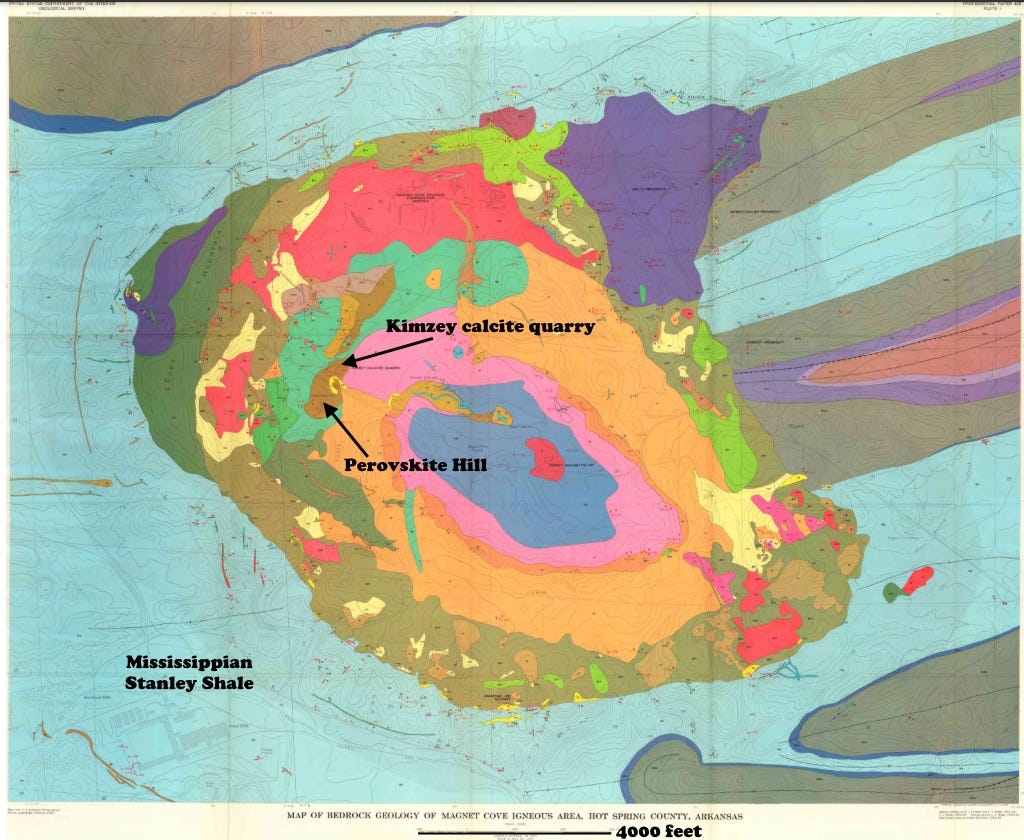
The intrusive carbonatite at Magnet Cove was emplaced about 95 million years ago (early late Cretaceous), probably along a deep-seated fault zone related to the opening of the Gulf of Mexico.
Most of the Magnet Cove perovskite comes from the appropriately named Perovskite Hill, where it is in the carbonatite, the related skarn, or both, and its crystals are often found loose in the soil. Perovskite is also found at the Kimzey Calcite Quarry within the carbonatite. For me walking into that quarry in 1969 was like entering a giant calcite crystal.
My specimens were all collected by well-known collector Clyde Hardin of Malvern, Arkansas, over time from the early 1970s to the 1990s based on his original labels which range from typed examples (early, the 1970s) to computer-printed (1990s). He also constructed complex pyramids of Styrofoam to support many of his specimens, which are generally quite small albeit usually very nicely formed crystals.
Perovskite crystals from Magnet Cove are usually sharp, looking like cubes or octahedrons or octahedrons with small cube terminations, but they are not any of those forms. Perovskite is orthorhombic, not isometric, but the crystal cell parameters are very close to the same, so the crystals have pseudo-cubic or pseudo-octahedral appearances.
Sometimes pseudo-cubic forms show penetration twins that are suggested to be especially common in niobium-rich varieties, which are called dysanalyte. I assume that’s the basis for Clyde Hardin’s labeling some specimens dysanalyte, but I don’t think there is any other reason short of analysis to suppose that any of these perovskite specimens is niobium rich.
Sometimes the pseudo-octahedral gray-black perovskite crystals sit on black octahedral magnetite crystals. At Magnet Cove, perovskite crystals are usually sharp and relatively smooth-faced, while the magnetite is often somewhat corroded, and perovskite is also not magnetic (though it can be challenging to be sure which part of a tiny amalgamation of crystals is, or is not, being attracted to a magnet).
A final interesting aspect of the perovskite is that often it alters from the original calcium titanate to anatase, one of the three common polymorphs of titanium dioxide (the others are brookite and rutile, both also common at Magnet Cove). That makes a brownish coating on the perovskite crystals. I haven’t analyzed any of mine but apparently it’s well established that the coating is indeed anatase.
Perovskite was discovered in Russia’s Ural Mountains in 1839 by German mineralogist Gustav Rose (1798-1873), who named it for Russian mineralogist Lev Perovski (1792–1856). Dysanalyte was thought to be a discrete mineral originally but is now considered to simply be niobium-rich perovskite. It was named from Greek δυσαυάλυτος, dysanalytos, hard to undo, because it was difficult to analyze.










Iron Hill (Powderhorn) is an important perovskite deposit in Colorado: https://pubs.usgs.gov/of/2009/1005/downloads/OF09-1005.pdf. Similar to Magnet Cove, it is a carbonatite complex. Perovskite is a potential source of titanium. When the property was owned by Buttes Gas and Oil Co. I was hired to work on developing processes to recover and convert the Perovskite to TiO2 pigment. Recovery of the rare earths was also investigated. I think that some crystals of perovskite have been found there, but all I ever saw was drill core which had no cavities where crystals could grow. The perovskite was mixed with carbonates, magnetite, and pyroxene and sort of resembled a marble cake. Like Magnet Cove, a significant quantity of the perovskite was altered to TiO2 (confirmed as anatase by XRD) but it is also mixed with other altered minerals and the mixture was referred to as leucoxene. It was higher in titanium than perovskite, but earthy and hard to recover. It was a very interesting project!
An interesting mineral, though my specimens come from the Oka Complex, Oka, Quebec, Canada. Children like the apparently perfect little black cubes. Right up there with little perfect pyritohedrons of pyrite and "Herkimer" diamonds.
These are niobium rich perovskites, but I have never seen any from the area labeled "Dysanalyte".
To my disappointment, upon analysis, none of the very sharp, black crystals collected have met the criteria to be labeled Latrappite instead.